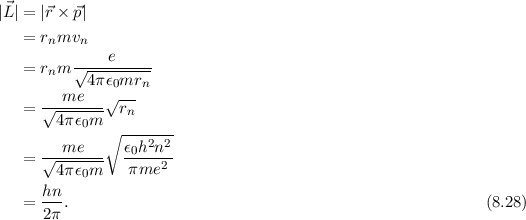Using Bohr's formula for energy quantization, the ionisation potential of first excited state of hydrogen atom is: . (1) 13.6V, (2) 3.4V, (3) 2.6V, (4) 1.51V

Using Bohr's formula for energy quantization, the ionisation potential of the ground state of Li^++ atoms is?

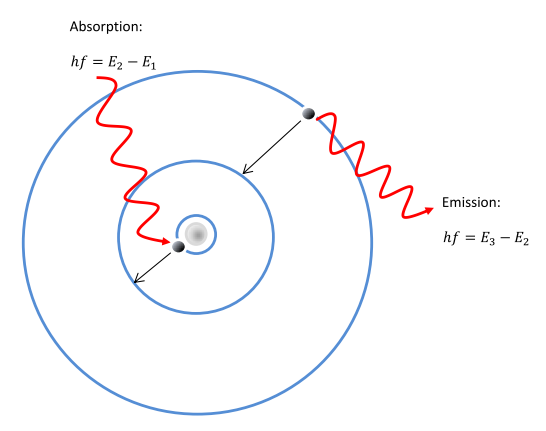
Would the Bohr formula for the H-atom remain unchanged if proton had a charge (+4//3) e and electron a charge (-3//4) e, where e = 1.6 xx 10^(-19) C. Given reasons for you answer.

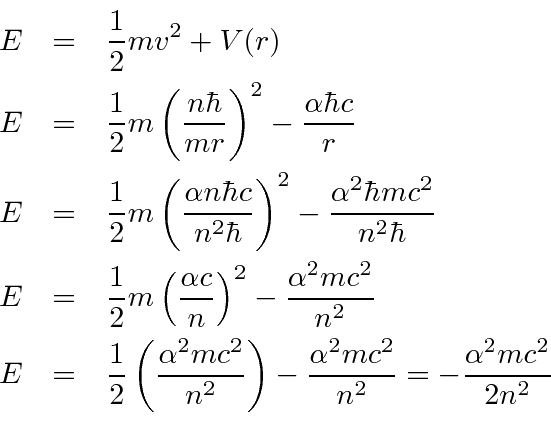

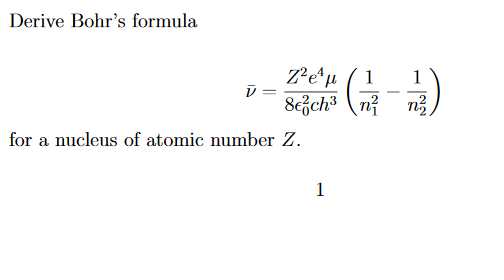



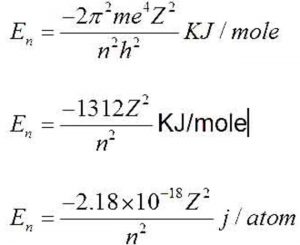
![Bohrsches Atommodell • Erklärung, Postulate, Formulierung · [mit Video] Bohrsches Atommodell • Erklärung, Postulate, Formulierung · [mit Video]](https://d3f6gjnauy613m.cloudfront.net/system/production/videos/001/833/2d5f6fe9ffd6a0af5733f881b21a0a9d4ccb0609/Bohrsches_Atommodell_Thumbnail.png?1628090256)